SARS-CoV-2 Reduction
- Authors
- Dana Yee, M.D.
- Facility
- Innovative Bioanalysis, California
- Download
- Full Report
PRODUCT BACKGROUND
Model tested was AC1500. Applicable models are AC1500 and AC1560.
The AutoClean 1500 is an automatic cleaning needlepoint ionizer. The compact unit mounts at the fan inlet of any air handling system, including RTU’s, PTACs, mini-splits, fan coil units and VRF systems.
OBJECTIVE
WellAir/Plasma Air supplied the AutoClean 1500 for testing purposes to determine efficacy against viral pathogens. This study evaluated the effectiveness of the AutoClean 1500 in its ability to reduce the viral strain referred to as SARS-CoV-2 within the air. Note, the AutoClean 1560 uses the same ionization component.
TEST METHOD
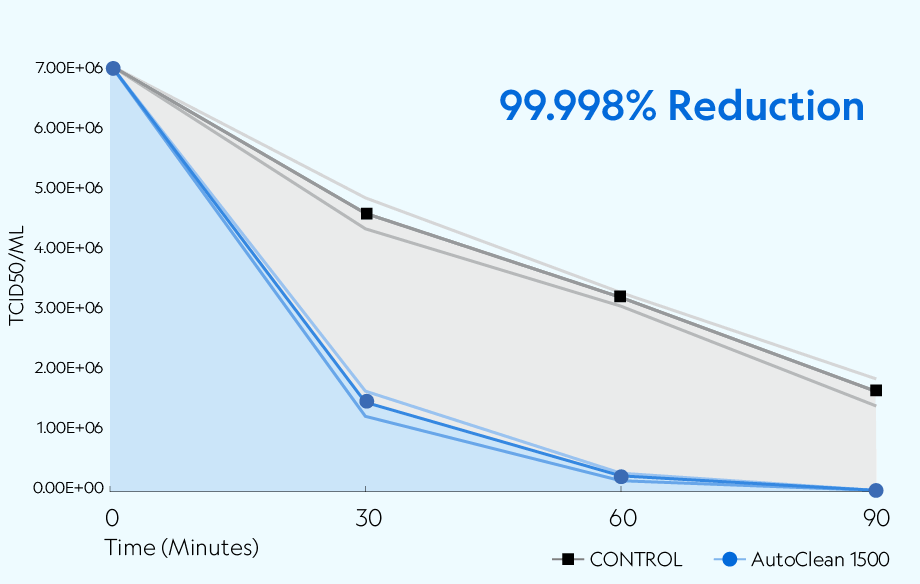
Bioaerosol Generation Test Substance: SARS-CoV-2 USA-CA1/2020 The nebulizer was filled with 7.01 × 06 TCID50/mL of SARS-CoV-2 in viral suspension media and nebulized at a flow rate of 1mL/min with untreated local atmospheric air. After nebulization, the nebulizer's remaining viral stock volume was weighed to confirm roughly the same amount was nebulized during each run. Bioaerosol procedures for the controls and viral challenges were performed in the same manner with corresponding time points and collection rates.
Testing Layout
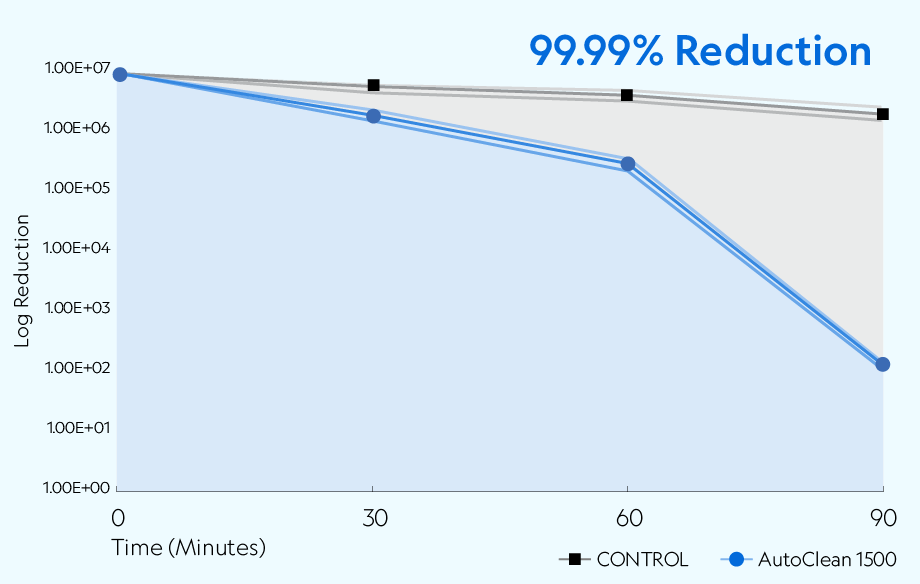
Testing was conducted in a sealed 20' × 8' × 8' chamber per Biosafety Level 3 (BSL3) standards. The overall dimensions of the test chamber provided a displacement volume of 1,280 ft3 (approximately 36,245 liters) of air. The chamber remained closed during testing, with no air entering or leaving the room. The chamber was equipped to create the necessary airflow to produce the required concentration of ions.* The temperature during testing was approximately 72 ±2°F (22.2 ±1.1°C), with a relative humidity of 37%. A 7.01 × 106 TCID50/mL of SARS-CoV-2 in viral media was nebulized into the chamber with mixing fans before collection. Air samples were collected at 30, 60, and 90 minutes after exposure.
Control Protocol
• Controls were conducted in duplicate without the device operating in the testing chamber to accurately assess the AutoClean 1500.
• Control samples were collected in the same manner and at the corresponding time points used for the challenge trial to serve as a comparative baseline to assess the viral reduction when the device was operating Experimental Protocol.
• Before the initial control test and following each experimental time point run, the testing area was reset, decontaminated, and prepped per internal procedures.
• 10 mL of 7.01 × 106 TCID50/mL SARS-CoV-2 in viral media was nebulized via the dissemination port into the room.
• After nebulization, the AutoClean 1500 ionizer was turned on via remote.
• The device was turned off at each pre-determined time point for air sample collection.
• Samples were collected after nebulization stopped (T-0) at the following time points with T equal to minutes: T-30, T-60, and T-90.
NOTE: Two controls and three viral challenges were conducted
CONCLUSION
The AutoClean 1500 demonstrated the ability to reduce aerosolized SARS-CoV-2 USA-CA1/2020 across all time points compared to the natural loss rate observed in the controlled setting. The device achieved greater than 99.99% (4 log) reduction of active viruses after 90 minutes of exposure.
The study focused on the impact the ionizer would have on a specific volume of space. Therefore, when applied to a different sized room, the results will scale and vary due to variables present, such as room size, occupancy rating, air movement, and more. Every effort was made to simulate a real-life situation and address constraints with the experimental design and execution while taking the proper precautions when working with a BSL-3 pathogen. These efforts are reflected in the meaningful recovery of the virus in the control test.